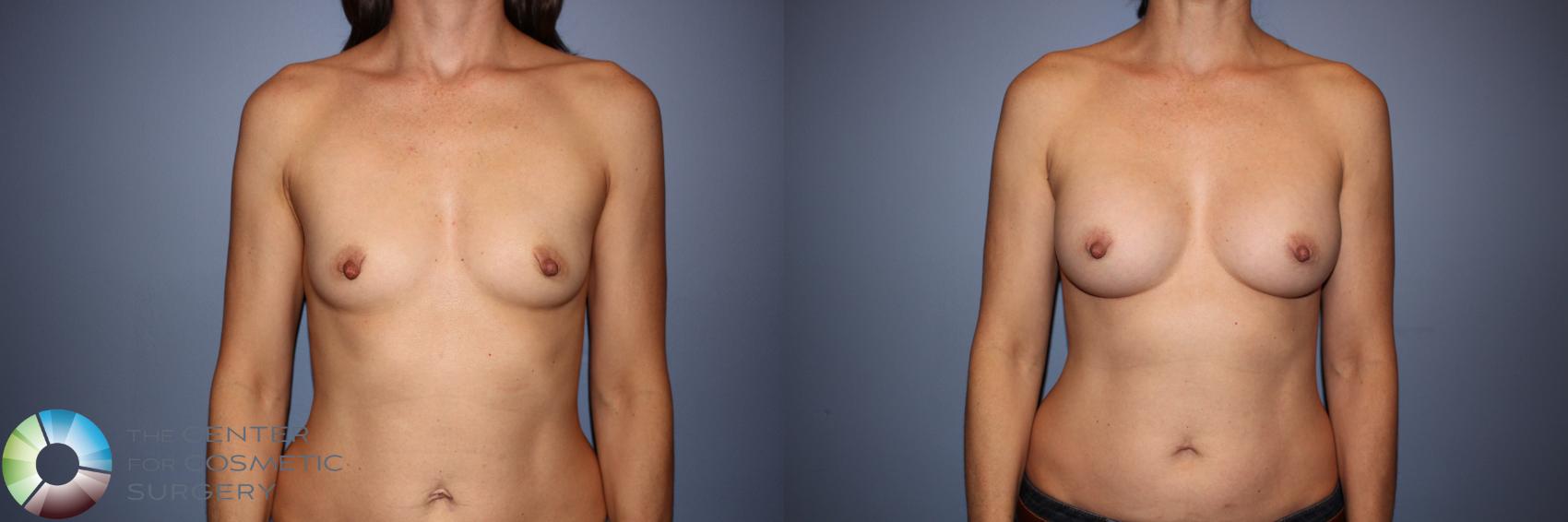
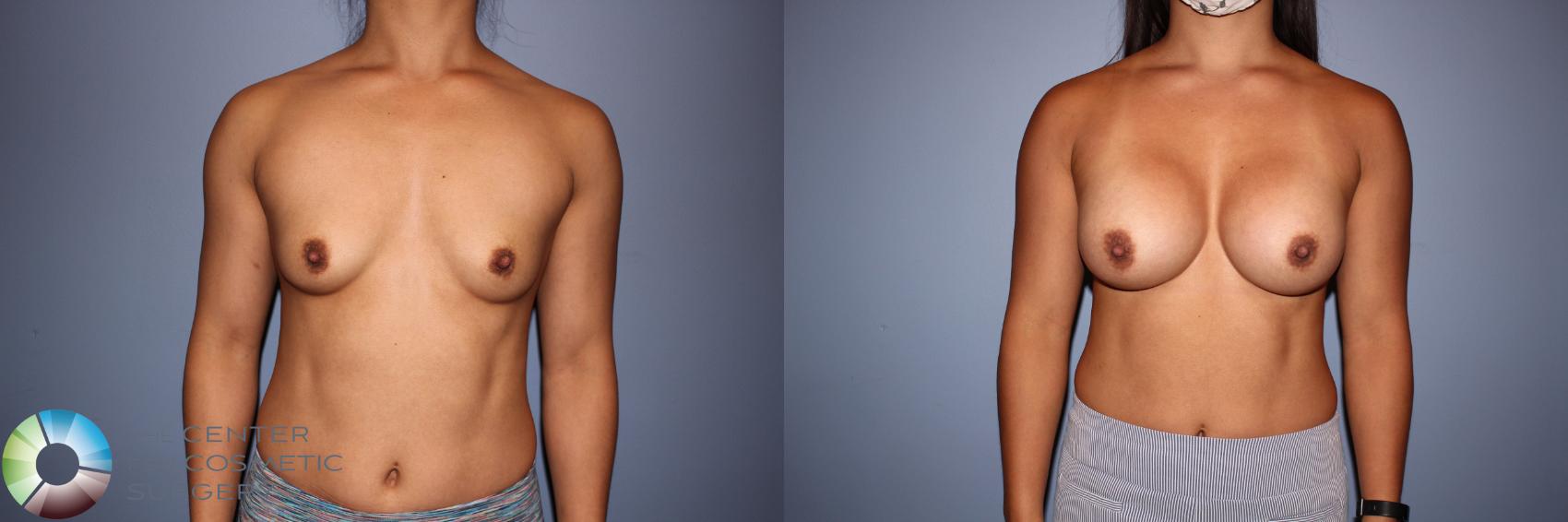
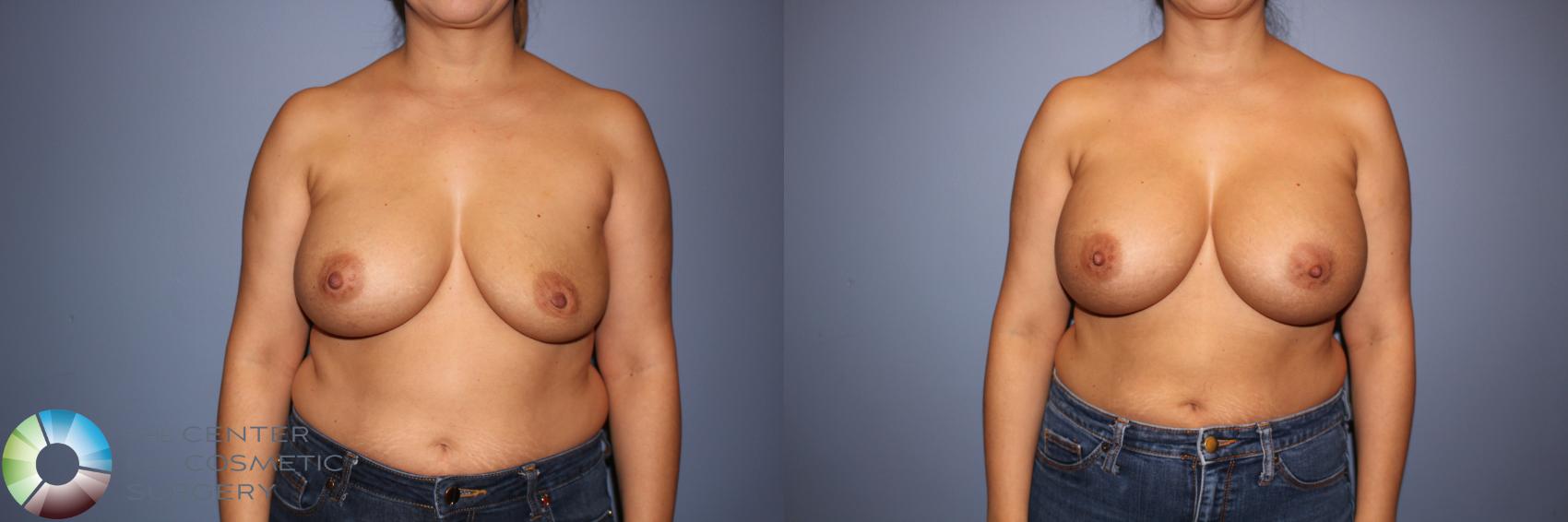
I was honored to be one of only 28 surgeons in the U.S. selected to participate in Motiva’s FDA clinical trial beginning in 2017. I performed 44 breast augmentations and revisions as part of the study and continue to follow these patients annually. At seven years, patient satisfaction has been excellent, and Motiva implants were approved in the U.S. in September 2024. Since that time, I have transitioned almost entirely to Motiva implants.
Breast augmentation and breast implant revision surgeries are a significant part of my practice. Having been in practice for nearly 25 years, I have seen a great deal of evolution in the breast implant space.
A brief review of where we’ve been can be helpful when examining where we are now.
The Saline Era
When I started practicing in 2001, a silicone ban was in place, forcing us to use saline implants for straightforward augmentations. We engineered a procedure that worked fairly well: subpectoral placement of slightly overfilled smooth saline devices could give very nice results in many patients.
In fact, many of my patients with saline implants placed early in my practice will still choose saline when the time comes to replace their devices.
In thin patients or those who had children, however, saline was a significant disappointment for many. Rippling, palpability (the ability to feel or detect the breast implant through the skin and tissue), and less natural movement were common in women with softer tissues, less breast tissue, and thinner fat layers.
The Return of Silicone
Silicone breast implants were reapproved in the U.S. in 2005 and 2006, opening up our implant choices. The silicone implants of that era were often better than saline but had their own limitations.
The gel was relatively liquid and less cohesive than modern versions. Rippling could still be an issue, implants did not hold their shape as well, and rupture rates were higher than desired.
Smooth vs. Textured Implants
Implant shells could be smooth or textured. Smooth implants were easy to use and forgiving but had higher rates of capsular contracture when placed above the muscle and were more prone to malposition.
Textured implants from Allergan and Mentor, and later Sientra, showed lower capsular contracture rates when placed above the muscle and improved positional stability. I used textured implants selectively with success, but still predominantly recommended smooth implants placed beneath the muscle.
Shaped and Textured Implants, BIA-ALCL, and the Industry Shift
In 2012 and 2013, shaped implants were approved in the U.S., first from Sientra and then from Mentor and Allergan. These were the first highly cohesive, or “gummy,” implants.
They were textured to prevent rotation. While rotation and malposition were uncommon, texturing introduced other risks, including seroma and double-capsule formation.
More significantly, textured implants were linked to Breast Implant Associated Anaplastic Large Cell Lymphoma, or BIA-ALCL. As awareness grew, Allergan removed its textured implants from the market, and use of textured implants declined dramatically. I now rarely recommend textured implants.
Modern Smooth, Cohesive Implants
As textured implants fell out of favor, manufacturers released more cohesive, smooth, round implants. These devices reduced rippling, improved form stability, and lowered rupture rates.
For many years, my standard approach involved smooth, moderately cohesive implants placed beneath the muscle, including Allergan Soft Touch, Sientra HSC+, and Mentor Boost implants.
Why Motiva Is Different
Motiva implants represent the newest technology available in the U.S. While other manufacturers offer reliable, time-tested implants, their designs are based on older shell and gel platforms.
Motiva’s proprietary SmoothSilk surface and sixth-generation gel were developed in 2010.
SmoothSilk Surface and Capsular Contracture
The SmoothSilk surface is neither smooth nor textured and received a unique FDA designation. This surface produces a lower inflammatory response and more predictable capsule formation.
Published studies show capsular contracture rates of approximately 0.5 percent, whether the implant is placed above or beneath the muscle. No other implant currently demonstrates this consistency. To date, there have been no reported cases of BIA-ALCL associated with Motiva implants.
Placement Flexibility
Because capsular contracture rates remain low regardless of placement, Motiva implants can be placed above or beneath the muscle with minimal concern.
Above-the-muscle placement offers faster recovery, no animation deformity, preserved chest strength, and reduced risk of muscle-related malposition. Today, I reserve submuscular placement primarily for very thin patients.
Rupture Rates
In the FDA trial, Motiva demonstrated a five-year suspected or confirmed rupture rate of approximately 0.5 percent in patients undergoing routine MRI screening.
Low capsular contracture rates also reduce mechanical stress on the implant over time, contributing to durability.
Ongoing Innovation
Establishment Labs continues to innovate. I was recently among a small group of U.S. surgeons given access to Preservé, a novel insertion technique designed to preserve breast anatomy and, in some cases, avoid general anesthesia.
Outside the U.S., Motiva also offers Ergonomix 2 implants, including versions with temperature-sensing technology.
Final Thoughts
Throughout my career, I have advised every major U.S. implant manufacturer. My decisions are guided by data and patient outcomes.
Given the consistently low capsular contracture rates above and beneath the muscle, low rupture rates, and focus on women’s health, Motiva implants are what I currently recommend for most patients seeking breast augmentation or implant revision.
To get started with breast augmentation, please request a consultation using the online form or call The Center for Cosmetic Surgery at (303) 278-2600.